The Shocking Discovery of Electrons: From Chemistry to Physics
Written on
Chapter 1: The Unsung Heroes of Chemistry
Throughout history, many renowned scientists have made significant contributions to chemistry, including Dalton, Lavoisier, and Mendeleev. While these names are often celebrated, others like Justus von Liebig, Joseph Gay-Lussac, and Jönny Berzelius also played crucial roles that are frequently overlooked. The electron, a fundamental particle in chemistry, is often recognized for its importance, yet few can identify the physicist who first discovered it.
The reason behind this oversight may stem from the fact that the individual responsible was a physicist. This marked a pivotal moment in scientific history, as the realms of chemistry and physics became intertwined. A solid understanding of electrons is essential for grasping the reactivity of elements, and this understanding inherently involves physics. As an organic chemist, I find this realization somewhat painful to admit, but it is true.
Now, let’s delve into the core of the matter. The world of science beckons!
Section 1.1: The Geissler Tube and Its Impact
In the age of discovery, advancements in scientific apparatus often precede groundbreaking findings. The Geissler tube played a crucial role in this context. The fascination with electricity eventually eclipsed the previous obsession with fire, leading to the discovery of potassium, a substance that reacts explosively with water. Gas discharge tubes became the new trend, much like neon signs, which are essentially sealed tubes containing gaseous elements with electrodes. When a high voltage is applied, the gas emits light. Early investigations were captivated by the colors produced, resulting in a plethora of perplexing research. Although beautiful, scientists like Michael Faraday found themselves bewildered, necessitating further experimentation and better tools.
Chemists proposed that a deeper understanding of these phenomena could be achieved by creating evacuated tubes, devoid of gas. However, fabricating such tubes was a skill only Heinrich Geissler (1814–1879) possessed. His evacuated discharge tube enabled a series of experiments that could not have been conducted with a standard tube, as the emitted glow would obscure the results. For instance, Julius Plücker (1801–1868) discovered that the fluorescent glow could be manipulated using a magnet, a finding that would not have been observable in a conventional gas discharge tube. Wilhelm Hittorf (1824–1914) and William Crookes (1832–1919) confirmed this observation and expanded upon it, demonstrating the shadow effect in their experiments, which led them to theorize the existence of rays of light.
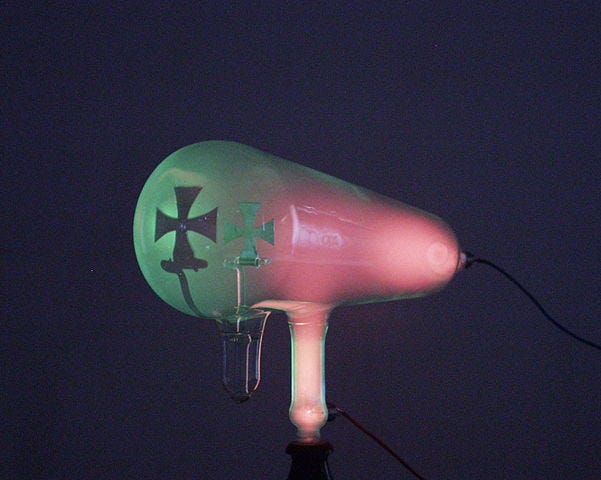
Section 1.2: Understanding Cathode Rays
A brief overview of electricity and its role in these experiments reveals that electrons flow from the cathode to the anode. In these discharge tubes, a significant number of electrons accumulate at the cathode, searching for a path to follow. Picture a scene from the movie 300, where Leonidas and his warriors trap a group of Persians against a cliff. The overwhelming force pushes the Persians to their doom—this analogy illustrates the behavior of electrons at the cathode. Without guidance, a free electron will wander inside the tube until it collides with something, resulting in the characteristic glow.
At the time, chemists and physicists were unaware of these dynamics, referring to this phenomenon as “cathode rays” because they appeared to emanate from the cathode, resembling light rays in their behavior. It was a source of confusion, as electrons are particles with mass, yet they were initially categorized as waves, similar to light. This misunderstanding deepened when Heinrich Hertz (1857–1894) demonstrated that cathode rays could penetrate aluminum and gold foil, seemingly supporting the flawed wave theory.
Fortunately, two critical developments in the late 19th century clarified this confusion. Firstly, advancements in tube design allowed researchers to create a focused beam of cathode rays in a more efficient vacuum. Secondly, Joseph John “JJ” Thomson (1856–1940) introduced his keen logic and innovative experiments into the discussion. Emerging from humble beginnings, JJ excelled academically and demonstrated that great scientists can arise from any background. His groundbreaking research at Cambridge in 1897 led him to:
- Establish that electrons are negatively charged particles.
- Calculate their charge and mass through a method he devised.
- Prove that the fluorescent glow is directly related to the presence of charged particles.
Section 1.3: The Legacy of JJ Thomson
JJ’s experiments revealed that particles emitted from the cathode were consistent regardless of the cathode's elemental composition. Furthermore, his calculations indicated that the mass of these particles was approximately 1/1000th that of a hydrogen atom. This finding was crucial in clarifying the debate over whether cathode rays were waves or particles, as the minuscule size of electrons allowed them to pass through foil without behaving like waves.
Ultimately, JJ’s research culminated in the validation of the existence of subatomic particles, which he termed “corpuscles.” This accomplishment earned him the Nobel Prize in Physics in 1906, along with 21 honorary doctorates and a knighthood. While "JJ" may be an endearing nickname, "Sir JJ" certainly has a more distinguished ring to it.
Interestingly, JJ was not fond of the term "electron," a name first introduced by George Stoney to describe a charged species. Moreover, this was not the last we would hear from him, as he would later propose one of the initial models of atomic structure. Yet, JJ Thomson lacks the same level of fame as John Dalton, perhaps due to his physicist status.
Despite this, my four years of PhD studies were spent contemplating electrons, even though I am an organic chemist. Therefore, I nominate Sir JJ to be featured on the Mount Rushmore of Chemistry Greatness.
Works Consulted:
Brock, William H. The Chemical Tree: A History of Chemistry. New York: Norton and Co, 2000.
Ihde, Aaron J. The Development of Modern Chemistry. New York: Dover Publications, 1984.
J.J. Thomson — Biographical. NobelPrize.org. Nobel Prize Outreach AB 2021. Mon. 16 Aug 2021.