Global Vaccine Development: A Comprehensive Overview of COVID-19 Efforts
Written on
Chapter 1: The Race for a COVID-19 Vaccine
As nations gradually emerge from prolonged lockdowns, the emphasis is shifting toward the creation of a worldwide vaccine to combat the pandemic, which has already affected over 5.7 million individuals and resulted in more than 357,000 fatalities. The World Health Organization (WHO) has cautioned that the crisis is far from over, with significant outbreaks still occurring in countries like Brazil, Russia, and India.
Governments reopening their economies must navigate the delicate balance between economic recovery and the ongoing health crisis. Recently, U.S.-based biotech company Novavax made headlines regarding its NVX-CoV2373 vaccine candidate.
This company has announced the commencement of its first Phase 1/2 clinical trial, enrolling human participants, with immunogenicity and safety results anticipated by July 2020. Novavax is leveraging its proprietary nanoparticle technology and Matrix-M adjuvant—previously utilized in its promising flu vaccine, NanoFlu—to develop an effective COVID-19 vaccine.
A noteworthy aspect of this initiative is the substantial $388 million funding granted by the Coalition for Epidemic Preparedness Innovations (CEPI), a collaboration of public, private, philanthropic, and civil organizations aimed at vaccine development. CEPI has provided smaller funding amounts, ranging from $1 million to $9 million, to other organizations like Inovio, Moderna, and CureVac working on coronavirus vaccines.
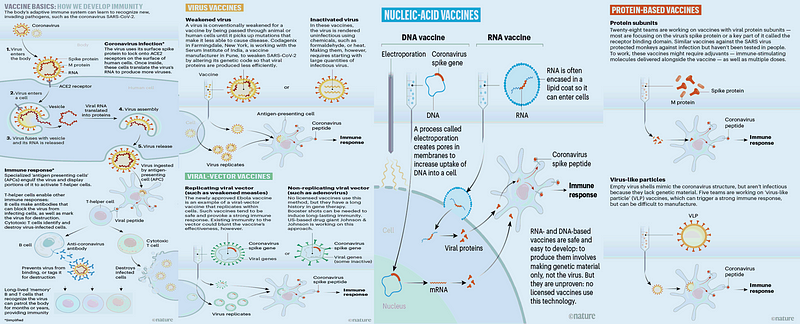
Chapter 2: Diverse Strategies in Vaccine Research
Various methods are being employed by medical researchers to create a vaccine. For instance, medical virologists at Frankfurt University hospital have been culturing SARS-CoV-2 cells since February. They have successfully identified existing drugs that seem to inhibit the virus's ability to replicate within host cells.
Virologists discovered that SARS-CoV-2 adopts a less aggressive strategy for reproduction compared to some other viruses. Instead of overtaking the host cell's production capabilities, it enhances the synthesis of proteins, utilizing the surplus for its replication. The researchers deduced that disrupting the supply of protein building blocks could halt the viral replication process. One compound identified, which is already available in existing medications, is the metabolism-inhibiting cancer drug WP1122.
Moreover, pharmaceutical giant Merck has recently entered the vaccine field by acquiring Themis Bioscience, based in Vienna, which is developing a vaccine. They are also collaborating with IAVI and Ridgeback Bio on vaccine and antiviral candidates, respectively.
According to WHO, the number of vaccine candidates in preclinical evaluation is growing, currently numbering 114. However, the following companies have ten vaccines in the clinical evaluation phase:
- CanSino Biological Inc./Beijing Institute of Biotechnology
- Moderna/NIAID
- Wuhan Institute of Biological Products/Sinopharm
- Beijing Institute of Biological Products/Sinopharm
- Sinovac
- Institute of Medical Biology/Chinese Academy of Medical Sciences
- University of Oxford/AstraZeneca/Serum Institute of India
- Novavax
- BioNTech/Fosun Pharma/Pfizer
- Inovio Pharmaceuticals
While a viable vaccine may still be months away, there are certainly encouraging signs on the horizon. In the meantime, it remains crucial to maintain physical distancing and uphold good hygiene practices to curb the spread of COVID-19. Stay safe and informed!
Stay updated with essential information—subscribe to my mailing list!