Exploring Heisenberg’s Uncertainty Principle with Python
Written on
Understanding the Heisenberg Uncertainty Principle
This series aims to apply the scientific Python stack to tackle real-world challenges, enhancing your skills through practice. By exploring a range of problems—from the common to the more intricate—utilizing libraries like NumPy, SymPy, SciPy, and Matplotlib, you can advance your understanding of scientific computing.
Daily Scientific Challenge
At the core of quantum mechanics lies the renowned Heisenberg Uncertainty Principle, a fundamental concept that dictates the limits of precision in measuring quantum states:
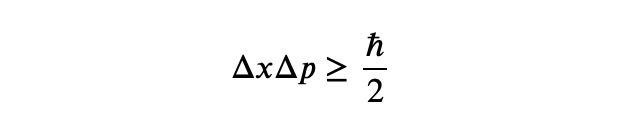
The "h bar" featured on the right represents Planck's constant, while ?? and ?? signify the uncertainties in a particle's position and momentum, respectively. The principle states that enhanced knowledge of one quantity leads to diminished knowledge of the other. Importantly, this is not merely a theoretical assertion! At atomic scales, any form of measurement inevitably disturbs the system, enforcing this limitation on uncertainties. Although the uncertainty principle is not a direct calculation tool, it underpins all phenomena at the quantum level. Today, your objective is to derive a lower limit for the quantum ground state of the harmonic oscillator.
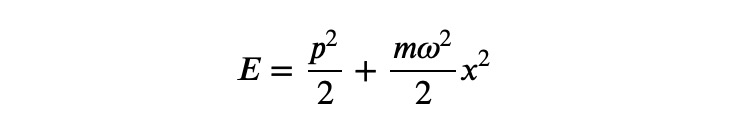
In this context, ?n is a positive integer, while ?, ?_0 are positive real constants. It's worth noting that in classical physics, the minimum energy would be ?=0, when both ? and ? are equal to zero. However, due to the Heisenberg uncertainty principle, achieving exact values for both quantities is impossible.
Implementing a Solution with Python
As is customary in SymPy, we initiate by defining our symbols:

The Heisenberg uncertainty relation can be expressed as:
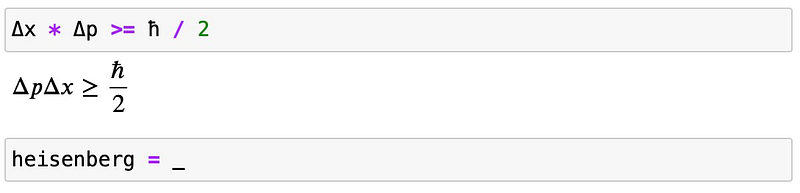
For the harmonic oscillator, we can represent it as:

Next, we will apply the uncertainty relation to substitute the position uncertainty:
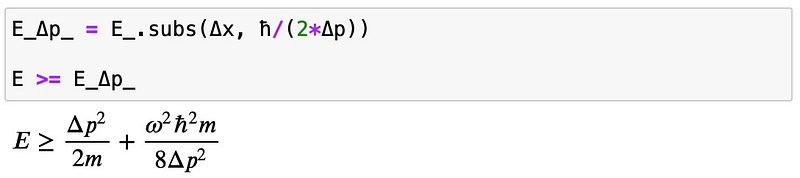
To find a lower bound for the energy, we seek the minimum and set the requirement:
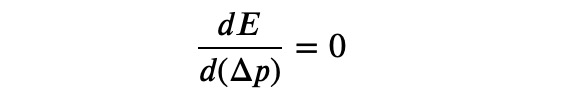
Continuing, we will solve for ??:
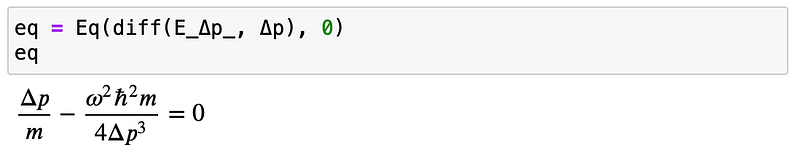
This results in the momentum uncertainty necessary to achieve minimum energy. We then substitute this value into our energy expression:

Ultimately, our conclusion is that the ground state energy must be at least ??/2. Remarkably, this is not just an estimation; it can be demonstrated that this represents the exact energy of the ground state.
Moving to a More General Potential
Now, let’s extend our findings to a broader potential scenario. We define the energy as follows:

By substituting the uncertainty relation:
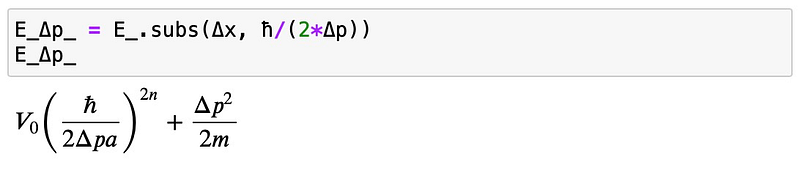
We will then investigate the minimum:

This time, we arrive at two potential solutions:

Since the uncertainty ?? must be positive, we can disregard the second solution.

Now, let’s insert the solution to ascertain the minimum energy:
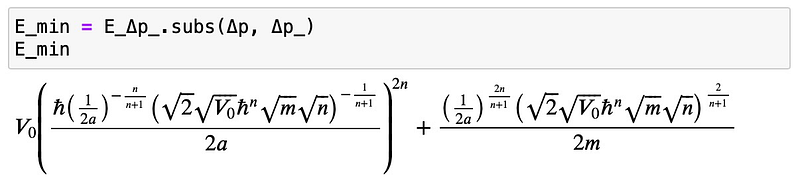
This scenario is relatively more complex, but by rearranging the terms for ?=1 (which simplifies to the harmonic oscillator case), we can observe the behavior as ?? approaches zero:
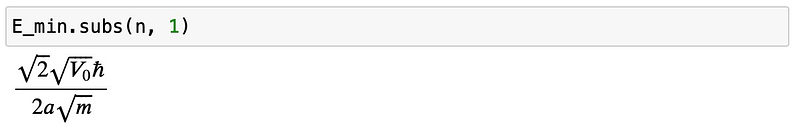
As ? represents the width of the potential, allowing ?? to approach zero leads to an increase in the minimum energy proportional to 1/?. In essence, confining the particle to a precise location results in a heightened uncertainty in momentum, subsequently raising the ground state energy of the system.
Relevant Videos
To deepen your understanding, check out these videos:
Calculating the Uncertainty with Python - YouTube
This video explains how to calculate uncertainties using Python, showcasing practical examples and code snippets.
Demystifying the Heisenberg Uncertainty Principle - YouTube
This video clarifies the Heisenberg Uncertainty Principle, breaking down its significance and implications in quantum mechanics.